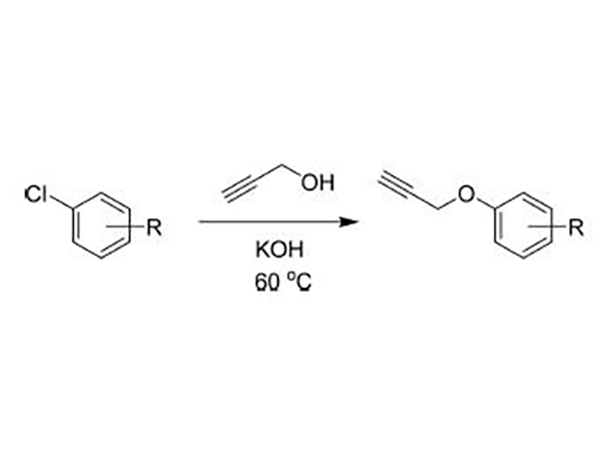
Products
Specializing in the production of propargyl alcohol, 1,4 butynediol and 3-chloropropyne
Propargyl will polymerize and explode
The initial process is based on propargyl alcohol as solvent, KOH as base, heating reaction to obtain the target. Reaction without solvent dilution conditions will be less impurities, the reaction is cleaner.
Considering the potential catalytic polymerization and explosive decomposition of the terminal alkynes, Amgen's Hazard Evaluation Lab (HEL) stepped in to perform safety assessments and assist in process optimization before scaling up to 2 liters of the reaction.
DSC test shows that the reaction starts to decompose at 100 °C and release 3667 J/g energy, while propargyl alcohol and KOH together, although the energy drops to 2433 J/g, but the decomposition temperature also drops to 85 °C, and the process temperature is too close to 60 °C, safety risk is greater.
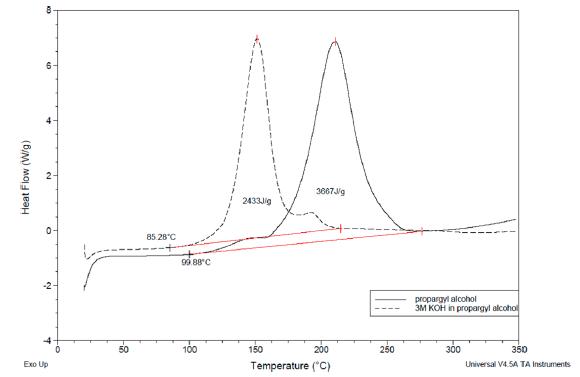
Yoshida Correction was used to calculate DSC data, and the results show that both propargyl alcohol and potassium hydroxide solutions are impingement sensitive and explosive.
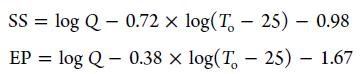
Kinetic regression using AKTS yielded a TD24 of 73.5 °C for pure propargyl alcohol and 45.9 °C for its 3 M KOH solution. Therefore, the system is not suitable for magnification.
Further test the reaction solution with ARC, a small heat release at 46 °C, adiabatic temperature rise of 6 °C, should be the target reaction heat release. At 76 °C, there was a strong heat and gas release, which directly caused the test tank to explode. It is further shown that the reaction is not suitable for amplification.
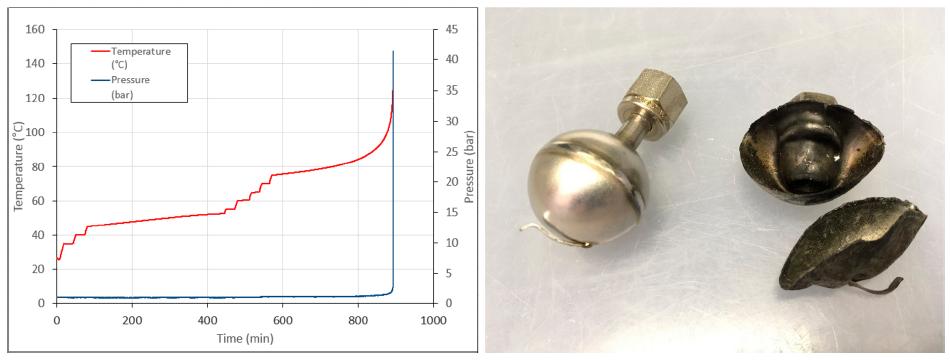
HEL and the team considered base change, but DSC tests showed that even the presence of base lowered the decomposition temperature of propargyl alcohol.
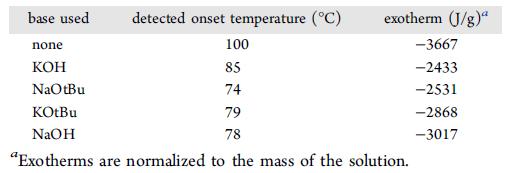
The screening experiments using alkali showed that the KOH reaction was good. Re-screening of solvents showed that dioxane was a better reaction. ARC tests showed that after the exothermic reaction of the target reaction, the temperature continued to rise to 200 °C and still no drastic decomposition was found. This condition can be safely scaled up.